MEDICAL DEVICE REGISTRATION

What is a Medical Device?
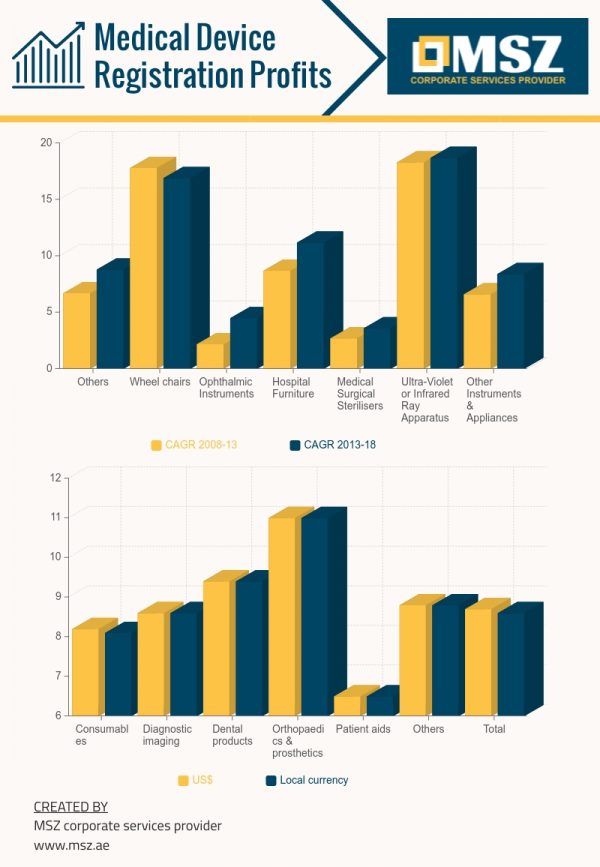
Products also including the accessories that are used in healthcare for the diagnosis, monitoring, prevention, or for the treatment of the illness or handicap excluding drugs. Medical devices can be dental products, consumables, diagnostic imaging, orthopedic and prosthetic products as well as patient aids.
Medical Device Regulations:
Each medical devices should be approved by the Ministry of Health Drug Registration and Control Department in the UAE. Imported medical devices require a pre-approval from the importation of the consignment that is given by the Ministry of Health.
Qualification of registration of Medical Devices:
An application willing to register a medical device in the UAE should be made by the device manufacturer or its distributor/local representative. The distributer/local representative should be formally authorized by the manufacturer for handling the application procedures, the manufacturer’s legal responsibilities as well as obligations about the place the medical device in the UAE market. The authorized distributor/representative should be available to associate between the Ministry of Health and the medical device manufacturer.
Required documents to the committee:
- All the certificates that are related to ISO 9001:2000 standards;
- the ISO 13485:2003 standard authenticated and attested Good Manufacturing Practice original certificate given by the related health authorities at the country of origin that must be authenticated and attested;
- Description of the device, intended use, using directions, contraindications, precautions, warnings;
Specifications of the material that is used in device packing and manufacturing; - Copies of documents and certification certifying ensuring to products standards, safety as well as quality systems in manufacturing and design;
- List of the countries where it is marketed as well as the details of the regulatory status;
- A summary of “compulsory” reported problems with a device since its introduction in the market;
- Risk assessment involving risk analysis, evolution as well as reduction measures;
- Complete detailed information on the safety studies that include clinical as well as pre-clinical studies, software studies, software validation studies where proper, and of the published reports dealing with the device;
Stability studies; - Price information, such as the ex-factory price
- Post-market requirements that are giving evidence of established process and systems for distribution records
These documents might be submitted in Arabic or English. The candidate should declare that all the documents that are submitted are true and that he/she will be completely responsible for the product as well as a post-market plan submitted for complaint handling and review and also that he/she is completely complying with the requirements of the Drug Control Department after placing the product in the market.
Main Competitors:
The UAE market is dependent on imports for medical devices; while the suppliers in the United States enjoy some benefits, the suppliers in Europe providers are invasively gaining market share with their proximity to the market as well as nearness to the market and viewed a better customer service.
Barriers:
The UAE market is dependent on imports for medical devices; while the suppliers in the United States enjoy some benefits, the suppliers in Europe providers are invasively gaining market share with their proximity to the market as well as nearness to the market and viewed a better customer service.
What is the Registration Process?
Presently the medical device regulations in the United Arab Emirates are based on European Union, Australian TGA as well as US FDA regulation. Products that are with the European Union, Australian, FDA, Canada’s TTP approval qualified for a shortened registration procedure in the UAE.
How to get medical device registration and approval in the UAE?
There are some steps to be followed to register your medical device. Get in touch with our experts who will help and assist you to get your medical device registered as well as approved in the UAE. Become a LICENSALE.COM user to get detailed device-specific compliance complete information for every market, also including the UAE, to expedite the planning of your medical device or IVD registration application.
What are Medical Device Regulations and Classifications in the UAE?
Regulatory Authority: All the medical devices are regulated in the Ministry of Health by the Drug Control Department.
Classification System: Medical devices are classified as per the European Union risk-based model into class
- I
- II(a)
- II(b)
- III
- IV
Timeframe: It takes between 8-9 months to get approval in the UAE.
The validity of the License: The license that is given in the UAE are valid for 5 years.
Authorized Representative: To register medical devices an authorized representative is required in the UAE.
Do contact us if you are looking to register your medical device in the UAE. Feel free to call us on Contact Us or drop us a mail at info@msz.ae

